
This page is intended to answer common questions about research ethics from medical residents in the UBC Faculty of Medicine conducting behavioural projects as part of their residency program (e.g., the Scholar Project in the Family Practice Residency Program).
If you are a medical undergraduate student in the UBC Faculty of Medicine conducting a project as part of your Flexible and Enhanced Learning (FLEX) course, please visit the Faculty of Medicine’s FLEX webpage. As this webpage is not intended to be exhaustive, if you have additional questions, please contact your Site Scholar Faculty or see the bottom of this page ("Who can I contact for assistance?") for links to your Research Ethics Board (REB).
Determining whether ethics review is required
This section describes the types of projects that require ethics review. It provides explanations for and examples of Quality Assurance/Quality Improvement (QA/QI) and Program Evaluation projects. It also describes when ethics approval is needed prior to accessing social media and medical records for research purposes. If you are still unsure whether your project requires review after reading this section, please reach out to your REB.
What types of projects require ethics review?
Projects that are carried out for “research purposes" and involve “human participants” or their data, may require ethics review. To help break this down, Tri-Council Policy Statement 2 (TCPS 2) Article 2.1 defines “research” as “an undertaking intended to extend knowledge through a disciplined inquiry and/or systematic investigation” where a “disciplined inquiry” refers to “an inquiry that is conducted with the expectation that the method, results and conclusions will be able to withstand the scrutiny of the relevant research community.” The TCPS 2 defines “human participants” as “individuals whose data, biological materials, or responses to interventions, stimuli or questions by the researcher, are relevant to answering the research question(s).” Note that the methods used and risk level do not have an impact on whether a project is considered research; it is the intention. For example, even if your project is low risk (e.g., an anonymous survey on a benign topic with a healthy adult population), if it is being carried out for research purposes (i.e., to systematically investigate a topic and add to existing knowledge on it), it would still require ethics review.
Examples of projects that require ethics review:
- Individual interviews with medical residents to explore the effects of sleep deprivation on concentration.
- Focus groups with international medical graduates about the barriers to integrating into the Canadian healthcare system.
- A retrospective chart review comparing clinical outcomes in diabetes patients treated with two different medications. Please contact your clinical REB if you are planning on conducting a retrospective chart review. For more information, please see CREB Guidance Note 14.6.4.
What types of projects do not require ethics review?
According to the TCPS 2, some projects that are carried out for “research purposes" and involve “human participants” or their data, do not require ethics review. These include those that exclusively use:
- Naturalistic observation of people in public places, provided you do not engage with those being observed and there is no reasonable expectation of privacy (e.g., counting from afar the number of people smoking in a non-designated smoking area).
- Secondary use of anonymous data, provided the data was originally collected without identifiers and the researcher does not know who the data was collected from (e.g., using anonymous survey data that was originally collected for a study on heart failure, in a new study on depression).
- Publicly available data (e.g., PubMed, Statistics Canada and other public websites that do not require creating a username and password).

The TCPS 2 also acknowledges that projects carried out for quality assurance/quality improvement (QA/QI) or program evaluation that use human participants or their data, do not require ethics review. For more information see, below.
When would my project be considered quality assurance/quality improvement (QA/QI) or program evaluation?
According to TCPS 2 Article 2.5, "quality assurance and quality improvement studies, program evaluation activities, and performance reviews, or testing within normal educational requirements when used exclusively for assessment, management or improvement purposes, do not constitute research for the purposes of this Policy, and do not fall within the scope of REB review." This is because a clear distinction is made between such activities and “research,” which Article 2.1 defines as, "an undertaking intended to extend knowledge through a disciplined inquiry or systematic investigation."
Note that the methods used, and risk level do not impact whether a project is considered research, it is the intention that matters. Although QA/QI and program evaluation activities often look “research-like” and may contain methods used in research studies (e.g., surveys, interviews, focus groups), because the purpose of these activities differs from the intent of research, they are outside the scope of REB review.
Typical medical resident projects that qualify as quality assurance/quality improvement (QA/QI) or program evaluation involve assessing a pre-existing program (e.g., an established wellness program for medical residents being run by the Faculty of Medicine) where the results generated will be fed back into the program and considered by those who oversee it. Importantly, the request to carry out the QA/QI assessment or program evaluation should come from the program itself, rather than from the medical resident. To help determine whether your project qualifies, you can consult the BREB’s QA/QI/Program Evaluation Checklist and contact your REB if you are still unsure.
Examples of projects that are considered QA/QI or program evaluation:
- The Faculty of Medicine has an existing module that is part of their teaching curriculum, and has asked you to survey medical residents to see how effective it is and if changes are needed.
- A medical clinic has asked you to survey patients at their clinic about how to change the layout of the waiting room to make it more comfortable.
- The College of Physicians and Surgeons has implemented a new treatment for diabetes patients and asked you to evaluate how many clinics are following this new protocol.
Examples of projects that are not considered QA/QI or program evaluation:
- You have created a new workshop, independent of the Faculty of Medicine teaching curriculum, to give medical residents practice with a particular clinical skill and decided to survey both those who complete the workshop and those who do not, to see if one group is more competent.
- You have independently decided to survey patients at two clinics with different waiting room layouts to see if there is a difference in patient stress levels.
- You have decided to conduct focus groups with medical residents to see if there is a correlation between stress levels and hours spent on call.
Note that if ethics review is not required, your project should still adhere to ethical principles. For example, projects involving an Indigenous community would require engagement and approval from the community; projects involving patients at a hospital would require operational approval from the associated health authority. You should also not refer to your results as “research” in any resulting reports or publications and should limit your discussion to the evaluation, rather than making broader statements about a particular phenomenon. If this will limit the scope of your project too much, you should consider submitting an ethics application.
Is ethics review needed to use social media for research purposes?
Social networking platforms like Facebook, Instagram and Twitter can be used for two distinct research purposes: recruitment and data collection.
If the platform will be used as a recruitment tool, your research would require ethics review. Since using social media to recruit participants has the potential to raise privacy issues, any postings used should include a disclaimer about the limits to confidentiality. Tip: Ensure you include the following statement on any social media recruitment postings, “If you choose to comment, like or follow this post, you will be publicly identified with the study.”
If the platform will be used to collect data, where signing up for an account or creating a password are not required to access the information being collected (e.g., tweets on Twitter), your research would not require ethics review as this information would be considered publicly available. If signing up for an account or creating a password are required to access the information being collected (e.g., posts on a private Facebook profile), your research would require ethics review.
For more information see Research Using Social Networking Sites.
As a clinician, do I need additional permission to use medical records for research purposes?
Yes.
Although medical residents have access to patient records for clinical purposes as healthcare providers, permission to use these records in for research is needed. If your project is behavioural in nature and you will be using a consent form to obtain permission from the patient to access their records, your application can be reviewed by the behavioural REB. If you will not be using a consent form to obtain permission from the patient (e.g., because you are doing a retrospective chart review, your application would need to be reviewed by the clinical REB. In all cases, ethics approval is needed before accessing medical records for research purposes and many organizations require that researchers provide their ethics certificate of approval as part of the application process.
Note that case reports, involving a retrospective analysis of one or two (at most) clinical cases, that have no research intention and will not be generalized beyond the cases discussed in them, do not require ethics review. If your case report does not require ethics review, you should still obtain consent from the patient(s) involved to include them. Please contact your clinical REB if you are planning on conducting a case report. For more information, please see CREB Guidance Note 4.4.2.
What to expect from the ethics review process
To help you plan ahead, this section provides an overview of the ethics review process, including how long it typically takes to fill out the application and to undergo ethics review. Shorter versions of the application for simple research projects, what it means to have your application “harmonized,” and when you should seek operational approval are also explained.
How long does it take to get approval?
Depending on the complexity of your study, minimal risk applications are typically reviewed and approved in 2-4 weeks. For more information about the review process see below.
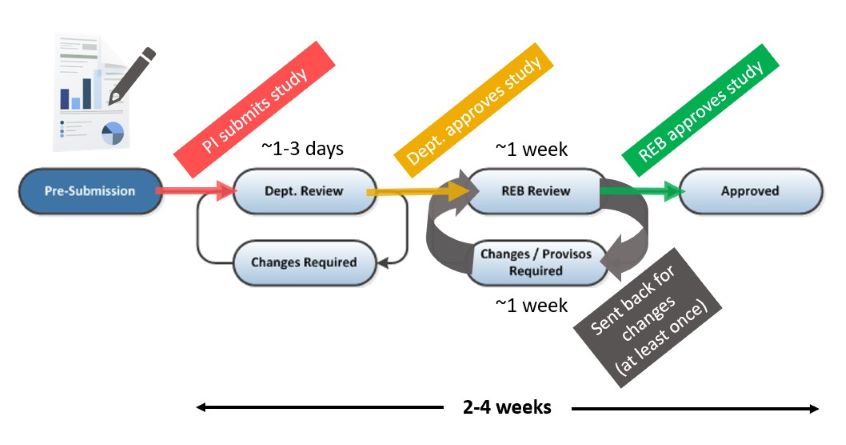
What does the ethics review process look like?
The review process consists of four phases, outlined in the flowchart below. While you are filling out the application, it is in “Pre-Submission.” Once your principal investigator (PI) submits the application, it then undergoes “Departmental Review” which takes 1-3 days on average. After the department has reviewed the application, it will either be sent back to you for changes via “Changes Required” or sent to the REB for “REB Review” which typically takes 1 week.
After the REB has reviewed the application, a list of changes or provisos will be sent back to you via “Changes/Provisos Required.” Once you submit your response to the provisos, the application will then be returned to the REB for “REB Review” which typically takes 1 week. After the REB has reviewed the application, it will either be sent back to you for additional changes via “Changes/Provisos Required” or be “Approved.”
The entire process takes 2-4 weeks on average, depending on the complexity of your project, how complete the application is when you submit it and how quickly you respond to the changes. For more information see the BREB Tip Sheet.
How long is the full ethics application?
The full application is 9 pages long. Given its length, you should not aim to complete the application in one sitting. As the application is different from a research proposal, it is also a good idea to discuss the questions with your supervisor before attempting to fill out the online version in RISe. For a full list of the questions, see the Word document version of the behavioural application.
Is there a shorter version of the ethics application for simple research?
Behavioural research: Shorter applications are available for behavioural research that only involves surveys or secondary use of previously collected data. Note that if your project involves surveys or secondary use of previously collected data plus another research method (e.g., interviews, focus groups or participant observation), it would not qualify for the shorter application. For more information about these shorter applications and how to complete them in RISe, see our Short-Form Guidance.
Shorter applications are also available for behavioural research that has already been approved by another Canadian TCPS2-compliant REB.
Clinical research: Shorter applications are available for clinical research that only involves retrospective chart reviews or secondary analysis of previously collected data or biospecimens.
What happens if my ethics application is “harmonized”?
An application is harmonized when the research being conducted involves sites that fall under two or more REBs that are part of Research Ethics BC . This can include:
- Having a study team member with an affiliation to a different health authority (HA) who is also carrying out their role in the study under that HA
- Carrying out recruitment, data collection or data analysis at a different HA
- Accessing medical records from a hospital under a different HA
If your application is harmonized, all of the REBs involved will be able to review it in RISe. This standardizes the review and helps ensure review quality and compliance with national policies. RISe automatically harmonizes your application based on your responses to the questions. All medical residents should select “UBC” as a study site in Box 4.2.A, regardless of whether their application is harmonized or not. If your study involves an HA and your application needs to be harmonized, you should:
- Select the HA in Box 4.2.A
- Check off the HA’s role in Box 4.2.D
- Include the institutional logos for UBC and the HA on the consent form
If your study involves an HA but will have no operational impact on the HA (e.g., there are no study team members acting under their HA affiliations, no HA staff or patients being recruited, and no HA office space or resources being used) your application does not need to be harmonized. Please check with your HA REB or UBC REB if you are unsure whether this applies to you.
Examples of projects that would be harmonized:
- Your supervisor is affiliated with Interior Health (IH) and is acting under their role at IH (UBC REB and Interior Health REB would review).
- You are accessing medical records from UBC Hospital and Victoria General Hospital for a chart review for research purposes (UBC REB and Island Health REB would review).
- You are recruiting patients from Vancouver General Hospital and Royal Columbian Hospital to complete a survey about a hospital run support group (UBC REB and Fraser Health REB would review).
When would my ethics application require operational approval?
Operational approval is separate from ethics approval. If your research involves utilizing health authority facilities, resources, patients or staff, you should contact the overseeing jurisdiction’s research office about whether operational approval is needed and how to apply for it.
Filling out the ethics application
How do I get started?
Ethics applications are submitted using UBC’s online platform, RISe. Before accessing RISe, you will need to create a RISe profile using your Campus Wide Login (CWL). Once you have a RISe profile, you can then log into RISe, click “Human Ethics” and start filling out the application, see below.
Remember to save your work as you go using the “Save” button.
Should I list myself as a principal investigator or co-investigator (Boxes 1.1 and 1.3)?
All medical residents involved in your project should be listed as co-investigators in Box 1.3 and identified as such in the consent form.
Only your supervisor can be listed as the principal investigator (PI) in Box 1.1, as they are the one who will assume responsibility for the research. The PI should also be listed in the consent form.
Medical residents in the Department of Family Practice whose supervisors are not UBC faculty, can list their Site Faculty for Scholarship as the PI in Box 1.1 and list their actual supervisor as an additional study team member in Box 1.5.
How do I know if my application is behavioural or clinical (Box 4.1)?
Behavioural research: These projects involve studying the relationship between people and their surroundings. Behavioural research at UBC is reviewed by the UBC Behavioural Research Ethics Board (BREB). Common methods include interviews, focus groups, surveys, questionnaires, observations, secondary use of data, behavioural therapy workshops and experimental coaching. They can still be about health and include health care providers and patients, provided the goal is not to modify direct patient clinical care (e.g., involving diagnosis, medication or treatment). Patient records can be accessed, provided consent to do so is obtained from the participant.
Clinical research: These projects intend to evaluate the effects of health-related interventions on health outcomes. Clinical research at UBC is reviewed by the UBC Clinical Research Ethics Board (CREB). Common methods include chart reviews, blood draws, clinical interventions, scans, drug administration, medical devices, biobanks, clinical data registries and analysis of laboratory, physiological, kinesiological or biological data obtained from physical interventions.
Research that includes both a behavioural method (e.g., survey, focus group, interview) and a clinical method (e.g., clinical intervention, blood draw, drug administration) should be submitted as clinical research.
How do I know if my application is minimal risk (Box 4.5)?
Most social science and behavioural research fall within the minimal risk category.
TCPS 2 Chapter 2, Section B defines “minimal risk research” as “research in which the probability and magnitude of possible harms implied by participation in the research is no greater than those encountered by participants in the aspects of their everyday life that relate to the research.”
The risk level of your project should be described in Box 4.5 using two variables, research risk and participant vulnerability.
For research risk, you should explain the reasonably foreseeable risks associated with your research methods (e.g., focus groups cannot guarantee confidentiality as you cannot control what the participants will do with the information discussed).
For participant vulnerability, you should explain the participant’s ability to safeguard their own interests within the context of the research project (e.g., healthy adults generally have a low level of vulnerability whereas patients or children may have a medium level of vulnerability).
Risk Matrix
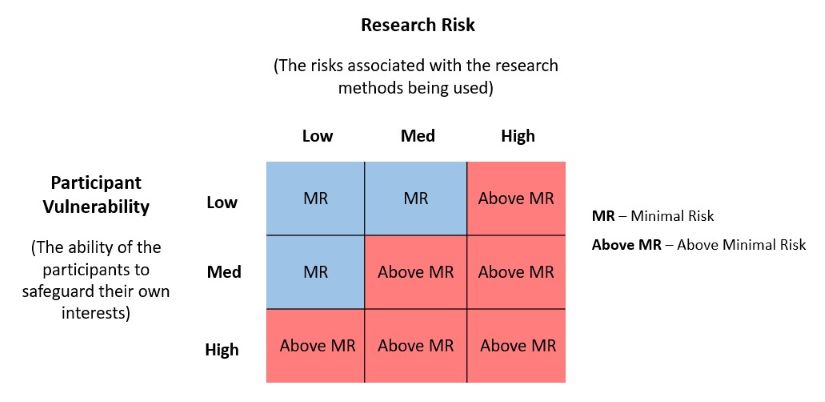
If your project falls within the blue section of the Risk Matrix, it would be considered minimal risk and if it falls in within the red section it would be considered above minimal risk. The risk level determines how your application is reviewed:
- Minimal risk applications (which are most medical resident projects) have no submission deadlines as they are reviewed in the order they are received, by one REB member.
- Above minimal risk applications have submission deadlines as they are reviewed at scheduled full board meetings, by multiple REB members. Full board meetings occur once a month at the BREB and twice a month at the CREB.
Examples of projects that are minimal risk:
- Interviews with healthy adults about how to design a user-friendly mindfulness app.
- Focus groups about perceived barriers to accessing substance use programs with health care providers who work in those programs.
- Surveys with medical residents about their comfort using POCUS (point-of-care ultrasound).
Examples of projects that are above minimal risk:
- Interviews with individuals who have eating disorders, about negative experiences they have had with their eating disorders.
- Focus groups about perceived barriers to accessing substance use programs, with individuals who are active substance users trying to access care.
- Surveys with youth about their experiences with cyber bullying.
Is there anything I should keep in mind during [with regards to] COVID-19?
If your research is taking place in person, please see the BREB COVID-19 Guidance for behavioural research and CREB COVID-19 Guidance for clinical research. If your research is taking place virtually (e.g., using Zoom), please see our guidance Using Zoom in Research.
Tips for filling out the application
General suggestions
Be consistent: Ensure the information in the application matches the study documents (e.g., the time to participate indicated in Box 6.1 and remuneration indicated in Box 6.5 should be the same as that mentioned in the consent form and recruitment material).
Double-check your work: As you fill out the application, your study procedures may change. Before you submit, you should go back and remove any information that is no longer relevant so it is clear to the reviewer what your project involves (e.g., you may start off wanting to do surveys with medical residents and their supervisors but later decide to only survey medical residents).
Provide enough detail: Clear step-by-step procedures should be provided when describing your recruitment, data collection and consenting procedures in Boxes 5.4, 5.6 and 6.5 (e.g., rather than just indicating that the focus groups will take place in Box 5.6, you should describe their size and composition, whether they will take place in person or virtually, and which team member will moderate them).
Consider power-over relationships: This type of relationship exists when the person conducting the research is in a real or perceived position of authority over the participants (e.g., medical residents would be in a dual role, as a healthcare provider and researcher, if they recruited their own patients). In these situations, the individual in the position of authority should not be involved in recruitment, data collection or consenting and should only have access to anonymized data, so that participants do not feel pressured to participate. This might be achieved through use of a third party who is not in dual role (e.g., a fellow medical resident who is a healthcare provider but not involved in the research).
Common errors made by medical residents
Below is a list of of application sections where errors are often made, with tips on how to complete them correctly. For a full list of administrative tips, see the Behavioural Application Checklist.
| Box | Topic | Tips |
|---|---|---|
| 1.1 & 1.3 | Study team | List your supervisor as the principal investigator (PI) in Box 1.1 and all of the medical residents on the study as co-investigators (Co-Is) in Box 1.3. For exceptions, see above, "Should I list myself as the principal investigator or a co-investigator?" |
| 4.4 | Peer review | Confirm that your supervisor has reviewed and approved the application. |
| 5.4 | Recruitment | Describe your recruitment methods in a step-by-step manner. If your study employs third party recruitment or snowball sampling, indicate that contacts will either: a) pass on recruitment material and allow those interested to contact you directly or b) obtain permission from those interested to share their contact details with you. |
| 7.3 | Research experience | Describe the relevant research experience of each study team member listed on page 1 as it relates to their role in the study, rather than just putting “MD.” |
| 8.5 | Data storage | Indicate that the data will be both password-protected and encrypted, the PI will remain responsible for the data and the data will be kept for a minimum of 5 years after publication. If the data will be destroyed, describe your methods for data destruction. |
| 9.2 | Consent form |
|
| 9.5 | Data collection | Attach your survey, interview and/or focus group questions. |
| 9.5 | Recruitment material | Attach your email scripts, posters, flyers, newsletters, letters of initial contact and/or social media posts. |
After you have ethics approval
The below describes things to consider once you have ethics approval in place.
Can I make changes to my application after it is approved?
If you wish to make any changes to your project after getting approval (e.g., add a new method of recruitment or data collection), you will need to submit a Post Approval Activity (PAA) for an Amendment to update the application and attach any new or revised study documents. These changes will need to be reviewed and approved by the REB before they can be implemented. See the PAA Amendment tutorial for step-by-step instructions.
Do I need to close my application once my study is finished?
You are responsible for submitting a PAA for Completion when your data collection is finished and you do not anticipate needing to recontact the participants in your study (i.e., for member checking or validation purposes). Step-by-step instructions for how to submit a Completion PAA are summarized below and described in this tutorial.
- Go to your application home page and click “New Post-Approval Activity.”
- Select “Completion of Behavioural Study” or “Completion of Clinical Study.”
- Complete the PAA coversheet. Note that per UBC Policy SC6, data must be stored for at least 5 years from the date of publication, within a UBC facility and remain under the responsibility of the PI.
- Once done, click “Save & Close” then click “Submit PAA” on the PAA Homepage.
Once the REB has reviewed and approved your PAA, they will terminate your application and you will be able to access it from your “Inactive” tab in RISe.
Resources, definitions and contact info
Where can I find consent form guidelines and templates?
Behavioural research: Consent Form Guidelines and Consent Form Checklist are posted here. If your research involves a survey and consent will be assumed if the participant completes the survey, rather than by obtaining signed consent, a consent cover letter can be used instead of a consent form. For what to include in the consent cover letter, see our Online Survey Guidance.
Clinical research: The BC Common Clinical Informed Consent Form Template is posted here.
What do "anonymous," "deidentified," and "anonymized" mean?
Sometimes medical residents use these terms interchangeably, but they mean different things when it comes to privacy. See TCPS 2 Chapter 5 for more discussion.
Anonymous: TCPS2 defines anonymous data as data that has "never had identifiers associated with it (e.g., anonymous surveys) and risk of identification of individuals is low or very low”. If the identities of the participants will be known to the researchers (e.g., interview participants or surveys that collect the participant's name), the data would not be considered anonymous.
Anonymized: TCPS 2 defines anonymized data as data that has been “irrevocably stripped of direct identifiers, a code is not kept to allow future re-linkage, and risk of re-identification of individuals from remaining indirect identifiers is low or very low.”
De-identified: De-identified data is data that has been collected with personal identifiers, but which have been removed and replaced with a code or unique identifier.
Confidentiality: The TCPS 2 Chapter 5 defines confidentiality as the ethical obligations "to protect information from unauthorized access, use, disclosure, modification, loss or theft.”
Other resources
TCPS 2
- TCPS 2 CORE Tutorial
- TCPS 2 Guidelines
Behavioural research
- BREB Guidance Notes
- BREB Application Guidance Notes
Clinical research
- CREB Guidance Notes
- CREB Application Guidance Notes
Who can I contact for assistance?
We suggest that you speak with your project supervisor first then, if you still have questions, reach out to your Research Ethics Board (REB).
For UBC affiliated REBs (including the UBC BREB, UBC CREB, UBC-O BREB, BC Cancer REB, Children’s & Women’s REB and Providence Healthcare REB) see the contact page on the Office of Research Ethics website.
For non-UBC affiliated REBs (including other post-secondary institution REBs and other health authority REBs) see Research Ethics BC.